T-Cell large granulocyte lymphocyte leukemia (T-LGLL), characterized by clonal expansions of cytotoxic T cells (CTLs), arises from polyclonal lymphoproliferative responses. Various triggers have been stipulated, ranging from autoimmune, viral or tumor antigens. However, one could also posit that an inherent propensity to overshooting CTL responses contributing to the initial clonal expansions or affecting normal termination of immune response might be at play. For instance, specific HLA risk alleles may be overrepresented or even diagnostic for certain autoimmune conditions. In contrast to the risk allele theory, which enriches haplotypes within the patient population, the concept of HLA evolutionary diversity (HED) posits that the structural divergence between alleles widen the recognition spectrum. HLA alleles also serve as ligands for killer immunoglobulin-like receptors (KIRs). Indeed, the KIR gene family, consisting of 15 genes and 2 pseudogenes, is responsible for regulating the activity of NK and a subset of T cells and thus may play a role in T-LGLL. The main KIR genes are classified as either activating or inhibiting, based on their ability to up or downregulate NK and T cells. As the KIR genes and their HLA ligands genetically assort independently of each other, KIR-HLA “mismatches” are possible.
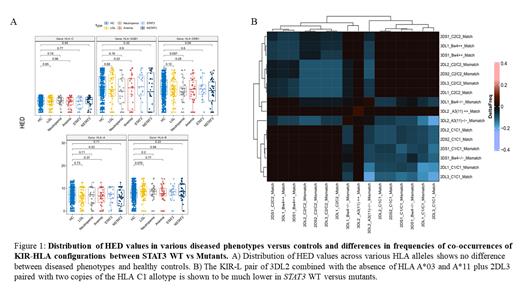
We analyzed HLA alleles in 91 T-LGLL patients vs 130 controls and investigated whether specific presentations of T-LGLL, namely neutropenia and anemia enrich for certain HLA alleles. We found that HLA-A*02 was more frequent in cases with neutropenia (59 vs 47%, p=0.045) and HLA-A*68 in patients with anemia (17 vs 8%, p=0.02) as compared to controls. Interestingly, HLA-A*02/HLA-C*05 was observed typically in STAT3 MT vs WT cases (22 vs 11%). Using the HLA calls, we calculated the HED for HLA class 1/2, but no increased HED could be asserted (consistent with the presence of risk alleles as described above).
We then explored whether specific KIR-HLA interactions enhanced an individual's risk for clonal CTL expansion and subsequent T-LGLL occurrence. As KIR gene frequencies can vary based on ethnicity, controls were chosen to match T-LGLL cohort (European ancestry). We applied T1K as a computational method for KIR genotyping and validated the calls by the 4 framework KIR genes (always present): 3DL2, 3DL3, 3DP1, and 2DL4. Along with the framework KIRs, there were no differences in the frequency of KIR genotype between T-LGLL and ethnicity matched controls. However, we found that the frequency of individuals with the 3DL2 gene and either HLA A*03 or HLA A*11 was significantly higher in the T-LGLL compared to controls (26 vs 8%, p = .006). Moreover, we found a higher frequency of 2DL3 combined with HLA C2/C2, (31 vs 14%, p=0.05) in T-LGLL patients with neutropenia. As this KIR-ligand (KIRL) configuration is a mismatch, the normally inhibiting receptor will have no effect on cell activity. Stratified by the presence of anemia, the LGLL cohort had instead a significantly higher proportion of 2DS2 co-expressed with the HLA C1 allele (75.68 vs 35.39%, p=.001), indicating activating genotype. We also found that the number of cases with the co-occurrence of the 3DL2 receptor and absent HLA A*03/A*11 ligands paired with 2DL3 and the HLA C1/C1 allotype was higher in STAT3 MT compared to WT (39.1 vs 16.7% p=0.06).
Finally, we attempted to estimate and compare the net KIR activity of T-LGLL patients and controls by creating a formula in which, for each individual, we subtracted the sum of the number of KIR/KIRL inhibitory matches from that of activating KIR/KIRL matches. Mismatches were also included either as activating or inhibiting depending on the KIR/KIRL configuration. Using this formula, we found that the net KIR average value for the control cohort was 0.85 compared to 0.69 for the T-LGLL cohort, indicating that the control cohort had slightly more activating KIR-ligand configurations. Even when mismatches were treated as neutral, the preexcitation balance was not changed in T-LGLL. These findings suggest that other factors may contribute to the baseline preexcitation levels of clonal CTLs in this disease.
In conclusion, our study of HLA and KIR-HLA interactions shows modest impact of KIR and KIR-ligand genotype on the propensity for overshooting clonal CTL proliferation and points towards other possible mechanisms such as hypersecretion of high affinity IL6R or other stimulatory factors.
Disclosures
Maciejewski:Novartis: Honoraria, Speakers Bureau; Alexion: Membership on an entity's Board of Directors or advisory committees; Regeneron: Consultancy, Honoraria; Omeros: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal